 WHAT WILL THEY THINK OF NEXT?
WHAT WILL THEY THINK OF NEXT?
There has been a lot of discussion lately about technology and vehicles. Much of this technology is finding its way to everyday vehicles, and more sooner than later, will be found on recreational vehicles. Some examples of these new tech-nologies are autonomous applications, hybrids with internal combustion engines and battery power, electric vehicles which are battery powered only, (brought forward into the headlines recently by Tesla, and look at what KTM is doing with battery powered motorcycles) and last but certainly not least, fuel cells.
Now it is probably going to be a while before a fuel cell ATV or Side by Side finds its way on to the show-room floor, but I think it is interesting enough to discuss this energy source at this time.
So, first and foremost, what is a fuel cell? Well, if you think of the automotive lead/acid battery you have in your car, you have the basic concept. The biggest difference is that when a battery is discharged, (dead) you recharge it. A fuel cell creates electricity primarily the same way, but it uses a fuel (hydrogen) to cause the reaction to create electricity. So as long as the fuel cell has hydrogen fuel, it will continue to cre-ate electricity. Let’s look a little deeper into how it works.
What is hydrogen? Remember the Hindenburg? Hydrogen is the simplest of all elements. It is number 1 on the “peri-odic table” which means it has 1 electron revolving around
a nucleus made up of 1 proton and 1 neutron. Hydrogen is by far the most plentiful element in the universe, making up 75% of the mass of all visible matter in the stars and galaxies. So…..this is good, we have a very large supply of the stuff.
Where do we find hydrogen? The fundamental question underlying the use of hydrogen as a fuel is, where do we get it from? Despite its abundance in the universe, hydro- gen does not occur freely on earth, as it reacts very readilywith other elements. For this reason, the vast majority of hydrogen is bound into molecular compounds such as water or H2O. (2 hydrogen atoms bound to 1 oxygen atom) Mostfuels like gasoline are hydrocarbon based fuels. Gasoline’scomposition is C8H18. So, we found where it is hiding, how do we extract it?
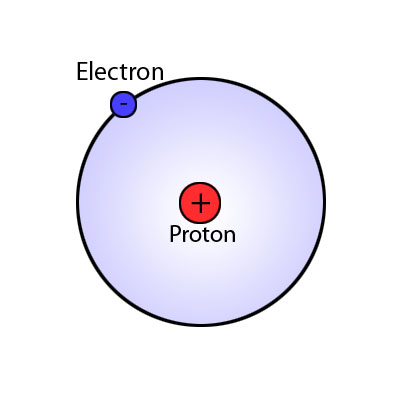
This is a basic hydrogen atom. One electron revolving around a nucleus that contains a single proton and neutron.
How do we manufacture hydrogen? To obtain hydrogen means to remove it from these other compounds. It could be water, which takes a lot of energy, or a fossil fuel which takes much lower amounts of energy. The process of extracting hydrogen from fossil fuels is called “reforming”. This may be considered a low cost method of producing hydrogen, but unfortunately, reforming emits pollutants and consumes non-renewable fuels. Extracting hydrogen from water is called electrolysis. Electrolysis can be entirely non-polluting and renewable, but due to the large amount of electricity required, the environmental impact of creating hydrogen in this manner is largely dependent on how the source power is generated. Once we get the hydrogen, what do we do with it?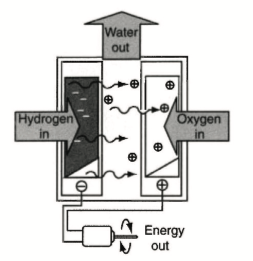
This schematic represents a very basic fuel cell. Hydrogen and Oxygen in, a reaction occurs causing electron flow and the electric motor rotates.
Hydrogen Storage. If extracting hydrogen is the toughest challenge when using it, then the second toughest challenge is how to store it. It can be stored as a liquid cryogenically, or as a high pressure gas. Depending on the application will determine how it is stored. The amount of hydrogen needed for a fuel cell is somewhat offset by the fact that a fuel cell uses hydro-gen more efficiently than an ICE (internal combustion engine)uses fuel, so less fuel is required to achieve the same result.Hydrogen also has the highest energy-to-weight ratio of any fuel. Gasoline has approximately 19,000 Btu’s per pound while hydrogen has 51,000 Btu’s per pound. OK, so now how does the hydrogen become power?
How does the Fuel Cell work? A fuel cell is an energy con- version device that converts the chemical energy of a fuel, di-rectly into electricity, without any intermediate thermal or me-chanical processes. Energy is released whenever a fuel reacts chemically with the oxygen in the air, whether it is combus-tively, as in an internal combustion engine, or electrochemically in a fuel cell. A fuel cell can operate without creating pollution, with the only by-products being pure water and heat.
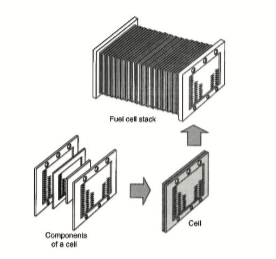
Here is how fuel cells are constructed into a fuel cell stack. Each cell will deliver a certain amount of voltage but combined together, a high voltage to drive a vehicle will result.
In a fuel cell there is no combustion, the reaction is purely chemical. Catalysts are used to combine the fuel (hydrogen) with oxygen. The reaction releases electrons, which is electri-cal energy. Fuel cells have no moving parts, and will continue to work until the hydrogen fuel supply is gone.
A single fuel cell produces a very low voltage, somewhere around 1 volt. To provide the amount of energy (power)needed to drive a vehicle, several hundred fuels cells are con- nected in series. This assembly of fuel cells is called a fuel cellstack. Since the cells are hooked in series, (let’s say 120 cells) the combined output would be approximately 120 volts. This voltage is high enough to power an electric motor on some type of vehicle. There are a different fuel cell designs, some not very practical or affordable for use in an automobile. It is worth mentioning that regardless of the type or design, they are all more efficient than the internal combustion engine.
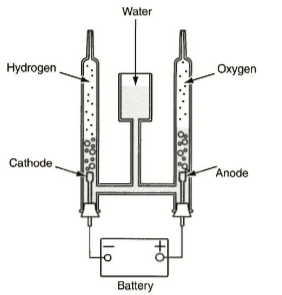
This picture shows that if a current is applied (in this case a battery) to water, the water will break down through electrolysis into hydrogen and oxygen.
The type of fuel cell currently being used in vehicle applica-tions is known as the PEM (proton exchange membrane). This type of fuel cell is favored by vehicle manufacturers because it allows for adjustable outputs which are necessary for variable driving speeds. It is also quite compact and it is capable of pro-viding high outputs. One of the biggest disadvantages of this type of fuel cell is that the membrane part of the assembly has to stay moist, so in cold climates, the moisture will freeze, mak-ing it very difficult to start operating. They (the manufacturer) are working on it though.
To investigate and learn more about fuel cells, go on to You Tube and search fuel cells. There are some great animations and short videos that will explain everything discussed in thiseditorial in greater detail.
Who would have thought a glass of water could power a car? What will they think of next?





